mechanisLet’s make things easy by breaking them into steps with visuals. Insulin synthesis, secretion, and function seem very hard to people but here I am making it easy by asking and answering the question which someone has in mind and this will engage you till to the last of the article because you will be bound to it. so let’s start with a question.
Have you heard about insulin synthesis, secretion, and function?
Yes, we have heard it but it looks like a scary phenomenon to remember.
Don’t worry, here I am making you involve till last when you dive into it.
The first question that comes to your mind.
I am confused about where you will start?

Don’t worry and don’t be confused, we will start from the pancreas.
What is the pancreas?
The pancreas is a double gland that works both as an endocrine as well as exocrine function.
Let me explain to you what is meant by endocrine and exocrine glands.
Endocrine gland:
Those glands which secrete their secretion directly into the blood and trigger the action.
Exocrine gland:
While exocrine are those glands that pour their secretion into a special duct and then transfer it to the target area for action.
Then how is the pancreas double gland and how does it secret insulin?
For this, you have to understand the endocrine structure of the pancreas. The pancreas has the following parts.
(I). Acinar cells:
The pancreas has acinar cells and associated ducts that help in digestion (not our topic). It is the exocrine part of the pancreas which makes up 99% of pancreatic mass.
(II). Islets of Langerhans:

Islets of Langerhans are small patches of islets that contain different types of cells and are the endocrine part of the pancreas. The name is given after being discovered for the first time by German physician Paul Langerhans in 1869. It forms 1% of the whole pancreatic mass.
Are the numbers of these islets known?
Not exactly the whole numbers are known but there are about 1-2 million islets of Langerhans in the pancreas. The pancreas has head, body, and tail parts in which the tail is mostly occupied by islets of Langerhans i.e. tail has the largest number of islets of Langerhans.
What different types of cells are there in islets of Langerhans?
Four different types of cells are there in the islets of Langerhans. These are:
(a). Beta (β) cells
(b). Alpha (α) cells
(c). Delta (δ) cells
(d). Pancreatic Polypeptide or PP or F cells
Can you explain them one by one?
Of course, this is my job here.
(a). Beta (β) cells:
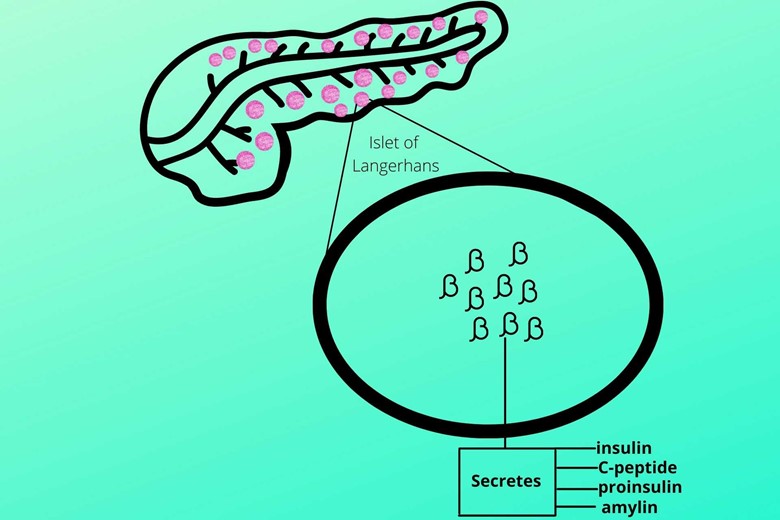
Beta cells make up about 60% of the islets of Langerhans and occupy their central position. Beta cells secrete insulin, C-peptide, proinsulin, and amylin.
(b). Alpha (α) cells:
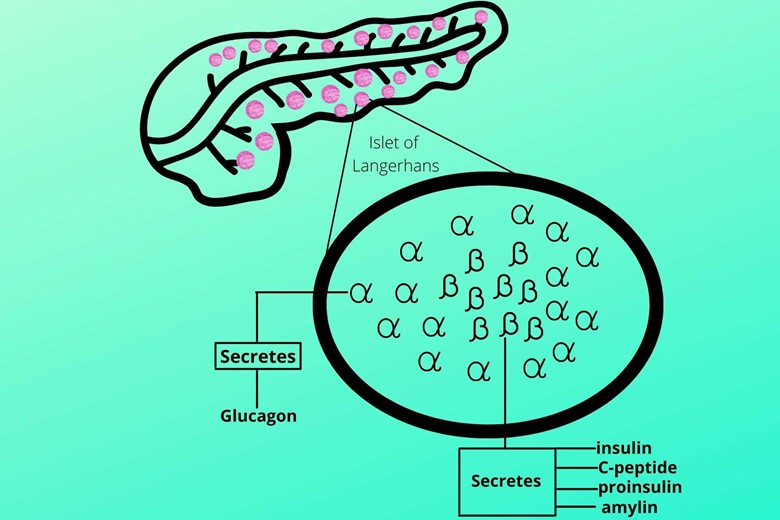
Alpha cells surround the beta cells and secrete glucagon. Glucagon converts the stored glycogen back to glucose.
(c). Delta (δ) cells:
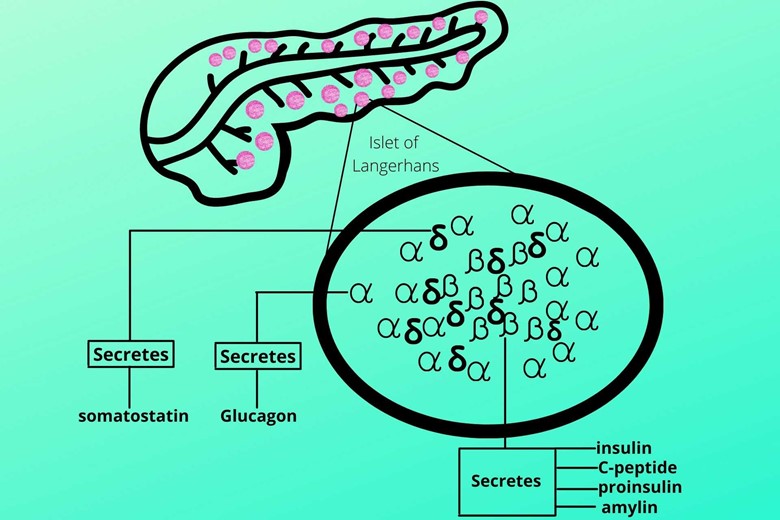
It secretes a hormone called somatostatin which inhibits the secretion of both insulin and glucagon. It is necessary for the control of nutrients in and out of circulation. It is dispersed between beta and alpha cells.
Some scientists consider somatostatin as a universal inhibitor of hormones.
(d). Pancreatic polypeptide or PP or F cells:
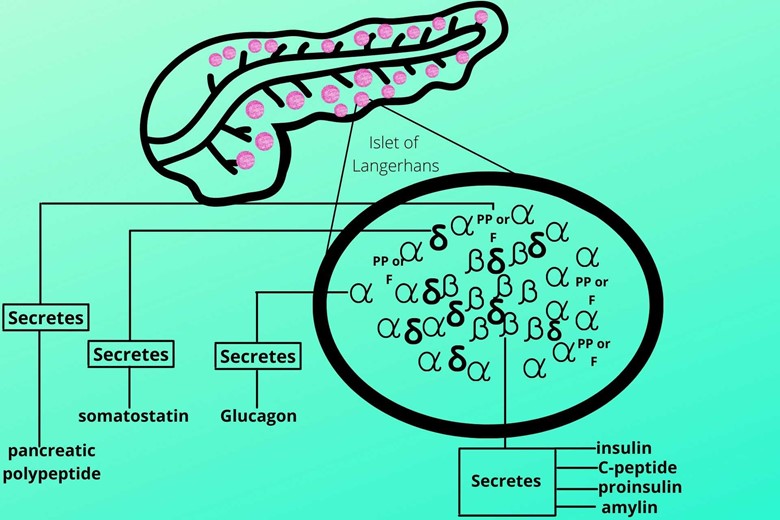
These cells secrete pancreatic polypeptide hormones which self-regulate the pancreatic secretion activities. It has also an effect on hepatic glycogen and gastrointestinal secretions.
That’s interesting.
Now we are clear about the endocrine structure of the pancreas.
What’s next?
Did you forget why we have discussed all these structures?
Oh, insulin synthesis, secretion, and function?
Yes.
First of all, I have to discuss the structure of insulin and how active insulin looks?
Insulin has a folded structure having:
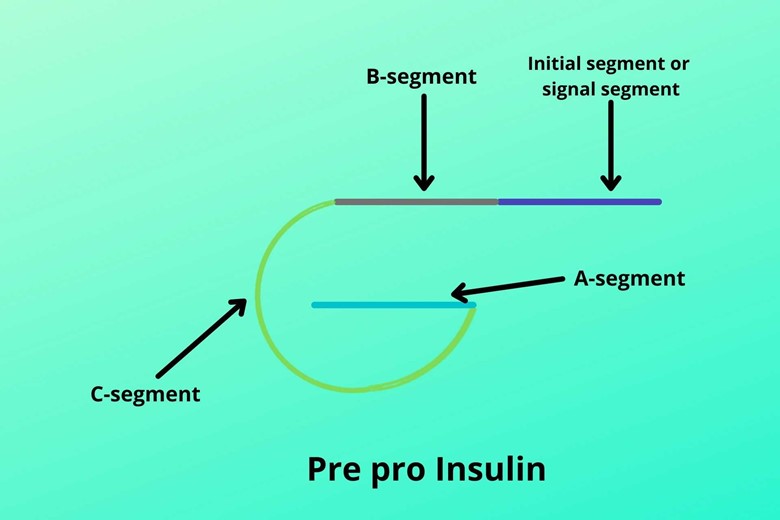
- Initial segment or signal sequence
- B chain
- C chain (it is the connector of B and A chains)
- A chain
This peptide chain is called pre-pro-insulin.
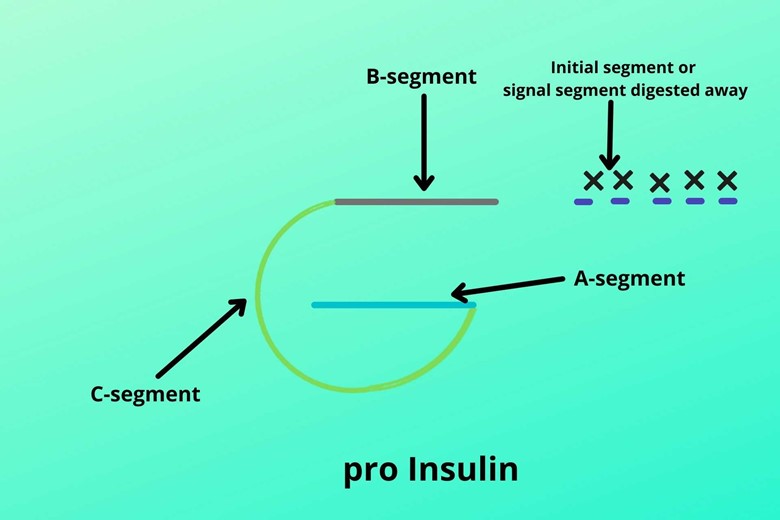
It is not active insulin so it doesn’t work. During the formation of active insulin, the initial segment or signal sequence is destroyed or digested away and the remaining part is then called pre-insulin.
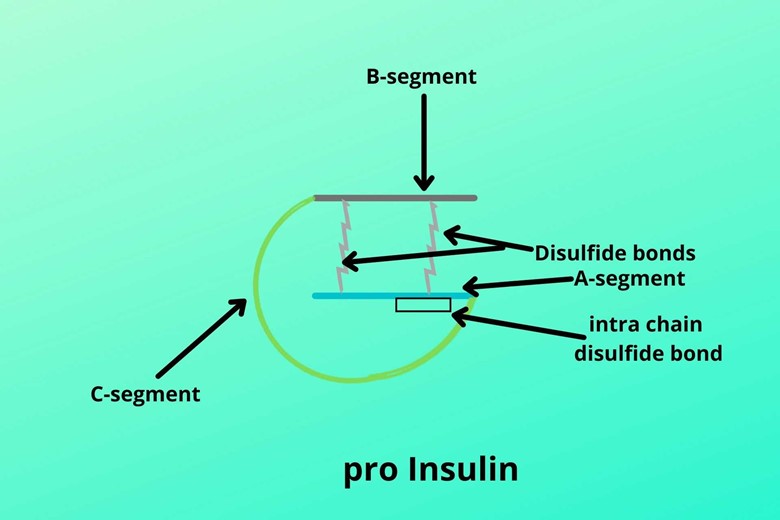
The further maturation process of insulin involves the formation of two cross-linked disulfide bonds between the B-chain and A-chain which hold them together and one disulfide bond in the A-chain (intrachain disulfide bond).
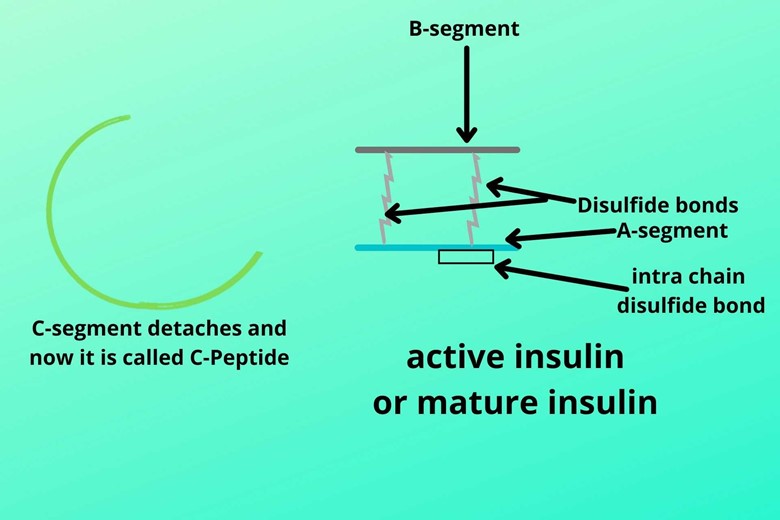
The C-chain gets broken from B-chain and A-chain. Now, this insulin is called active insulin or mature insulin while the C-chain is now called C-peptide.
Is C-peptide produced with insulin?
As pre-pro-insulin is converted into active or mature insulin and C-peptide so it means both C-peptide and active insulin are produced in the very same amount i.e. equimolar amount.
But what is the function of C-peptide?
The C-peptide is a marker for insulin secretion because insulin is 60% destroyed during the first pass to the liver. The enzyme which breaks the structure of insulin into separate B and A chains is the insulinase enzyme. The insulinase enzyme is present in the liver and kidney.
So during certain conditions, C-peptide is measured to find the level of insulin released.
Sometimes people inject a high dose of insulin exogenously to get hypoglycemic attacks for some purposes like insurance or other benefits. The exogenous (market) insulin does not contain C-peptide so to know whether it is true or fake just measure the C-peptide level. In this case, the C-peptide level will be low despite the high insulin level because as I already mentioned that exogenous insulin does not contain C-peptide.
Did you get the structure of insulin?
Yes, it is pretty clear now.
Now we are going to explain how insulin synthesis starts?
As you know insulin is a peptide hormone so it involves all the steps of protein synthesis.
You mean the first step is transcription?
Exactly, the first step in protein synthesis is transcription so it is also the same for insulin which is a protein.
So from which chromosome the transcription starts?

The chromosome has both long and short arms but the short arm of Chromosome number 11 has the gene for insulin.
(i). Unzipping of DNA:
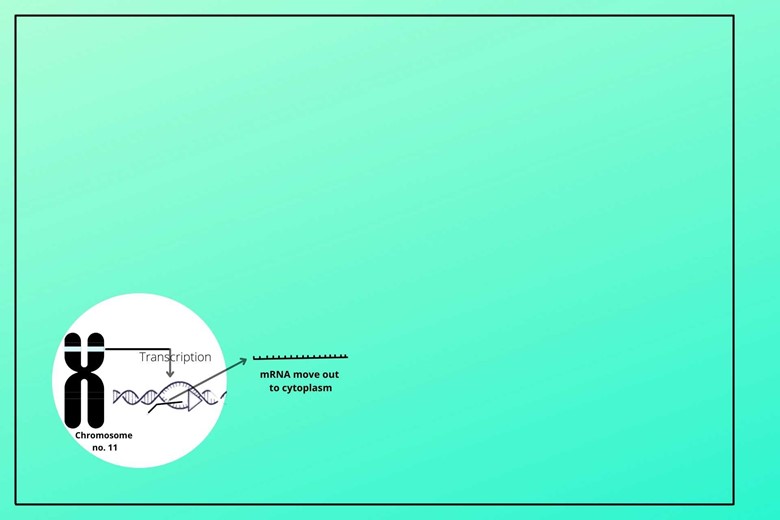
The DNA unzips and messenger RNA from that (for insulin) gene is copied and moved to the cytoplasm for further action.
(ii). Attachment to the ribosome:
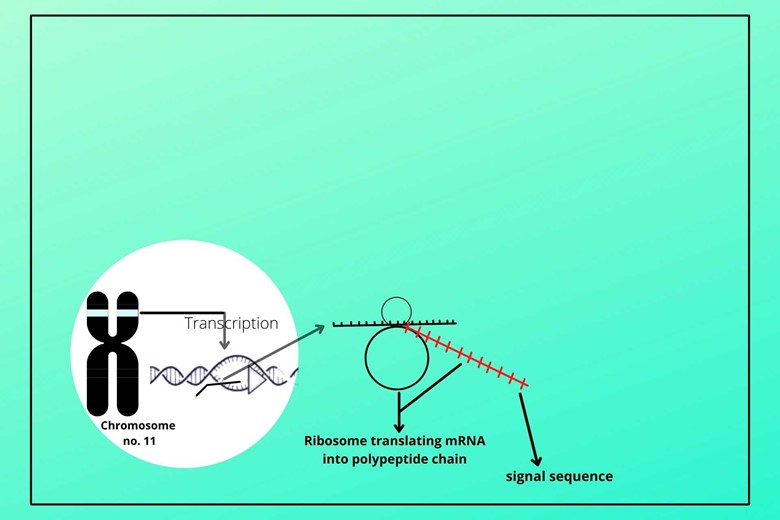
The messenger RNA (mRNA) gets attached to the ribosome in the cytoplasm and translates the code of DNA to an amino acid by transferring RNA. As translation starts the initial translation results in the signal segment of insulin.
(iii). Dragging of mRNA to Rough Endoplasmic Reticulum:
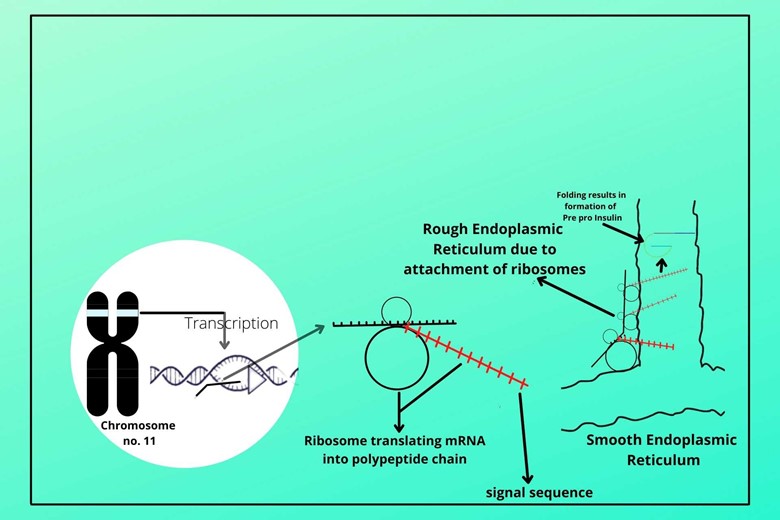
The signal segment drags the mRNA and ribosome to the Endoplasmic reticulum and makes it rough endoplasmic reticulum. The other mRNA has synthesized a longer peptide chain than the signal sequence which is dragged to the lumen of the endoplasmic reticulum where it gets folded to form pre-pro-insulin.
(iv). Digesting/breaking of the signal segment:
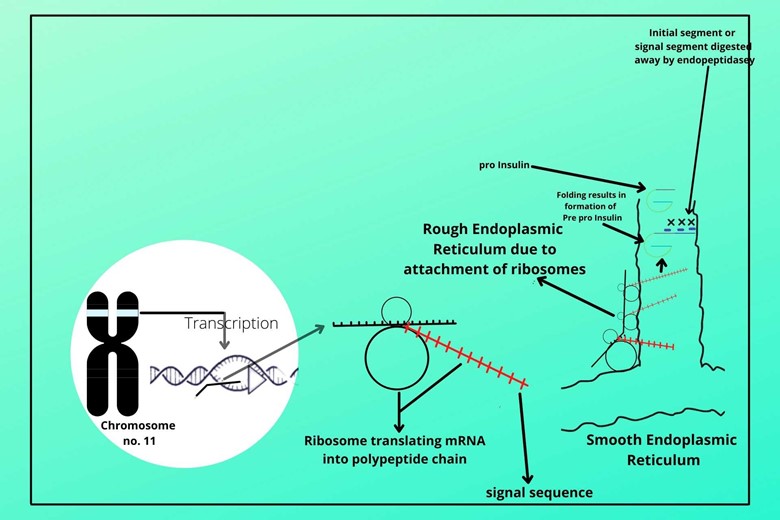
There is an enzyme called endopeptidase which cuts and digests the signal segment of pre-pro-insulin in the lumen of the endoplasmic reticulum to form proinsulin.
(v). Formation of vesicle and budding off of proinsulin:
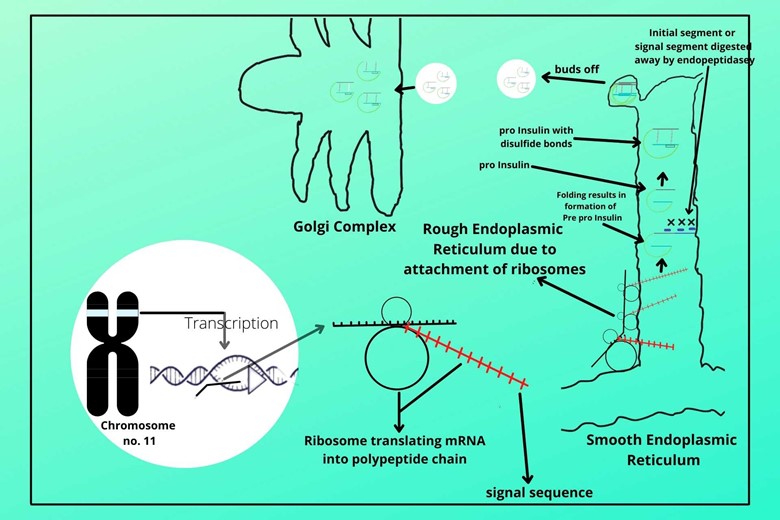
The B-chain and A-chain of proinsulin form cross-disulfide bonds. The endoplasmic reticulum membrane forms a vesicle where proinsulin enters and buds off from the endoplasmic reticulum and moves to the Golgi complex/apparatus.
(vi). Formation of active insulin and C-peptide:
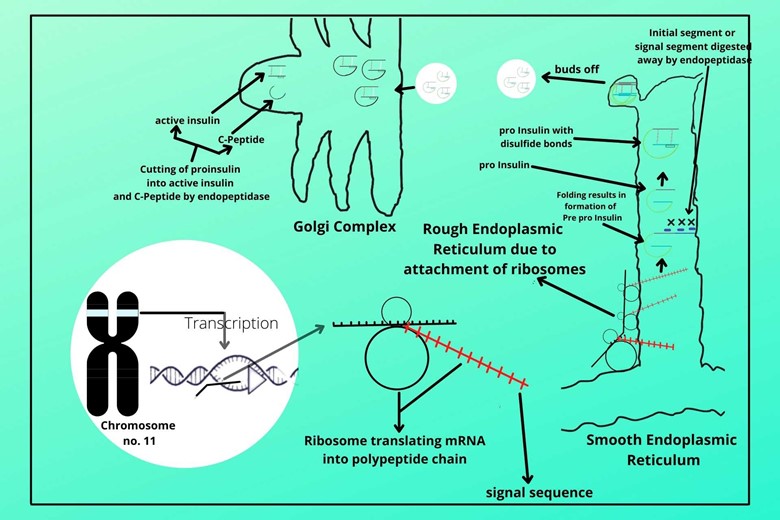
In the Golgi apparatus, another endopeptidase enzyme comes which cuts the C-chain from B-chain to A-chain. The B-chain and A-chain are already linked by a disulfide bond and now it is the biologically active and mature insulin while the C-chain is called C-peptide which is produced in equal amounts with active insulin because whenever proinsulin is cut, it converts not only to active insulin but also to a C-peptide too.
(vii). Budding off from Golgi Complex:
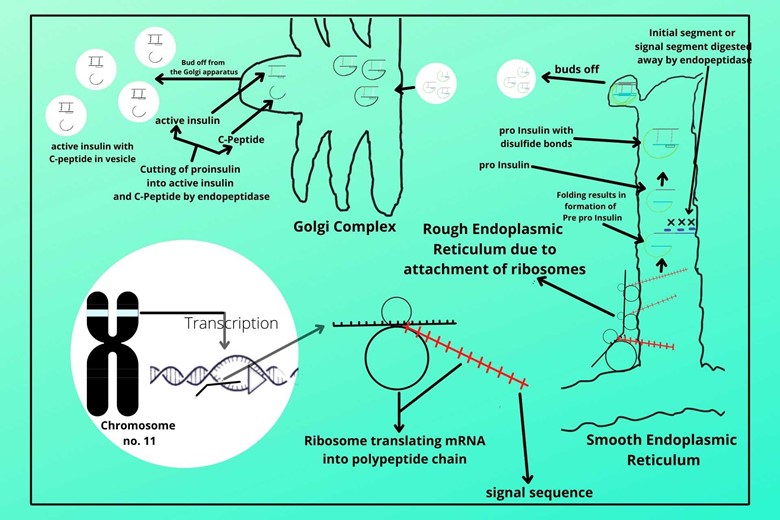
The active insulin and C-peptide are packed in another vesicle which is the bud off from the Golgi apparatus. The endopeptidase action of cutting proinsulin to active insulin and C-peptide still continue in the vesicle. And when the secretory vesicle secretes its secretion, besides active insulin and C-peptide it also contains a little amount of proinsulin which is not yet cut to active insulin and C-peptide.
(viii). Storing of active insulin and C-peptide:
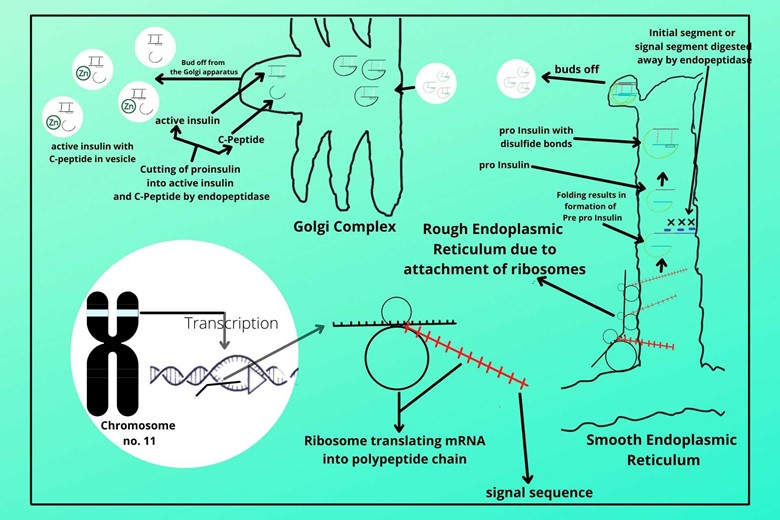
In the last, the active or mature insulin and C-peptide are stored in a vesicle with the association of Zinc. Insulin is stored in the hexameric form in vesicles. Hexameric form means that one zinc molecule has six molecules of insulin.
But remember when it is released into the blood it may be in the form of dimeric or monomeric form.
But which is an active form in blood?
Monomeric is the biologically active form of insulin in the blood.
These are the steps involved in the synthesis of insulin and its storage.
Now guess what is the next process of stored insulin?
I guess their secretion.
That’s correct.
Now it is the process of how beta cells know that the level of glucose is raised?
That is a good question.
The glucose entry starts from the gastrointestinal (GIT) system to blood circulation during eating. Now from here, the glucose moves to a beta cell.
Wait, but how is the blood entry pattern in the islets of Langerhans?
That is also a very good question. As we have discussed that the beta cells are in the middle of the islet of Langerhans, and the blood directly enters to beta cells first in the islet. The blood directly enters the beta cells in the center of islets of Langerhans through arterioles, then move to the periphery of the islet through fenestrated capillaries which spread in all directions of the islet of Langerhans and is finally collected through venules to outside.
So what are the benefits of this pattern of circulation?
When blood is moving to the periphery, the beta cells release their secretion, insulin, to the blood too which has an important function.
The alpha cells around the beta cells get exposed to insulin so alpha cells will not release glucagon.
This regulation in which one cell regulates the secretion of nearby cells is called paracrine regulation.
Now coming back to the topic of how beta cells know the raised level of glucose and how exactly insulin secrete on the molecular level?
I will explain in steps so that it is easy for you. For convenience, the steps are divided into a story that has two parts, insulin secretion, and insulin transport.
- Story part 1
- Story part 2
1. Story part 1:
Story 1 has the following events.(I). Glucose transporter type-2 (GLUT-2)
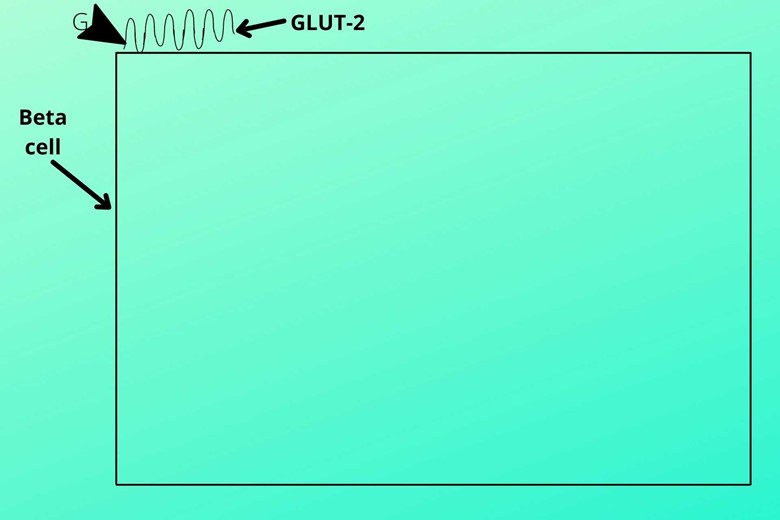
The blood with glucose enters to move to beta cells of islet Langerhans and enters through glucose transporter type-2 (GLUT-2) bet cell. GLUT-2 is a special glucose transporter that binds the glucose to itself and then attaches to the membrane of beta cells.
(II). Flipping in of glucose transporter type-2:
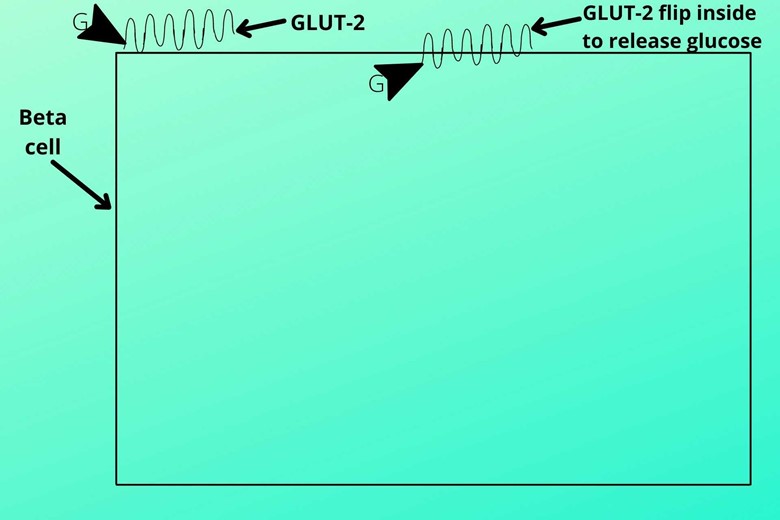
After the attachment of GLUT-2 along with the glucose molecule to the membrane of the beta-cell, it flips in the cell and will release the glucose molecule inside the cell.
After releasing the glucose molecule inside the cell the GLUT-2 again flips out and binds to another glucose molecule and the process repeats.
The GLUT-2 moves glucose molecules from higher concentration to lower concentration as after making carbohydrate-rich food the glucose level is raised in the blood than the beta islet of Langerhans so it will transport the glucose molecule inside the beta-cell.
Is this special glucose transporter only present in beta cells?
No, this GLUT-2 is also present in the liver, intestine, and kidney.
LIKe term will remind you where GLUT-2 is present besides beta cells.
L liver
I intestine
K kidney
(III). Phosphorylation of glucose:
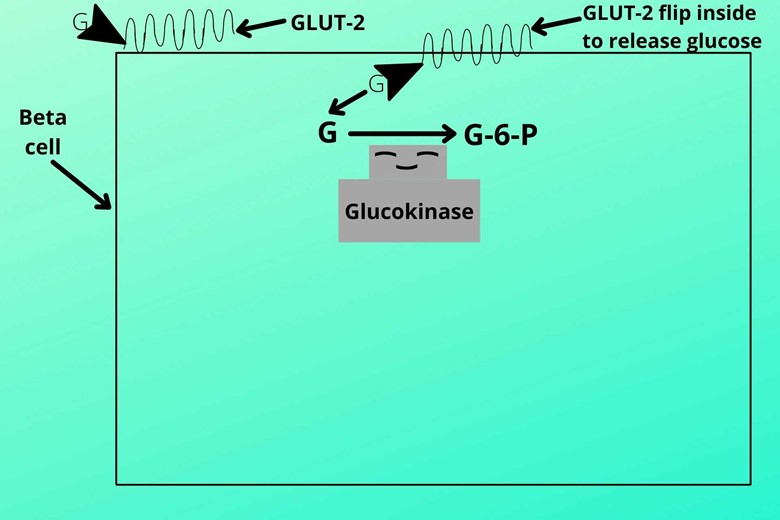
When glucose molecule enters the beta cells it is phosphorylated (addition of phosphorus) into glucose-6-phosphate by an enzyme called glucokinase
Why it is necessary to phosphorylate glucose?
In GLUT-2 the two means, bidirectional. In simple words, it means that GLUT-2 can enter as well as move out the glucose molecule from beta cells. So after phosphorylation, the glucose molecule gets so charged that it is not able to escape from the cell and is trapped in the cell.
(IV). Glycolysis:
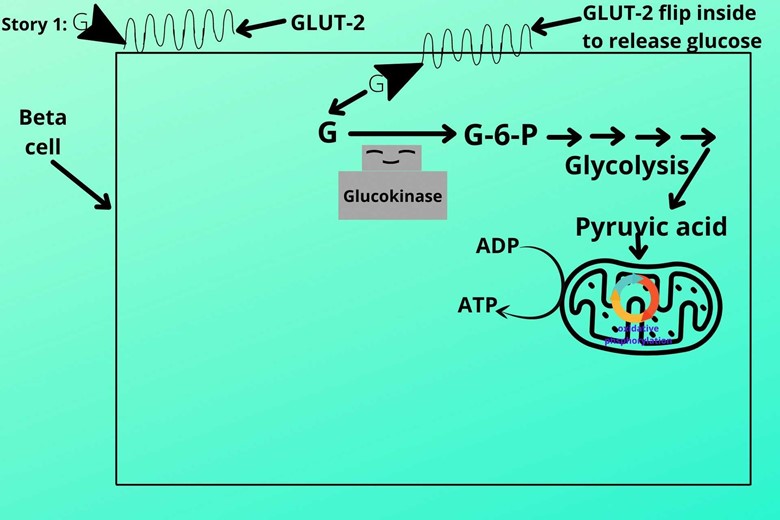
The cell then moves the phosphorylated into the pathway of glycolysis which ends in the production of pyruvic acid. The pyruvic acid is then transported to mitochondria and will pass through two processes that are:
- Tricarboxylic acid cycle (TCA CYCLE)
- Oxidative phosphorylation
(V). Conversion of ADP to ATP
During the TCA cycle and Oxidative phosphorylation, the energy is produced which converts the ADP to ATP so the level of ADP gets reduced and the number of ATP moves up in the cell.
Story part 1 ends here, now we are going to explain part 2 of the story.
(2). Story part 2:
Story part 2 has the following steps. Remember that these all steps are going in the beta cells of islets of Langerhans. Here we will discuss the potassium and insulin relationship.
(VI). Potassium (k+) channel:
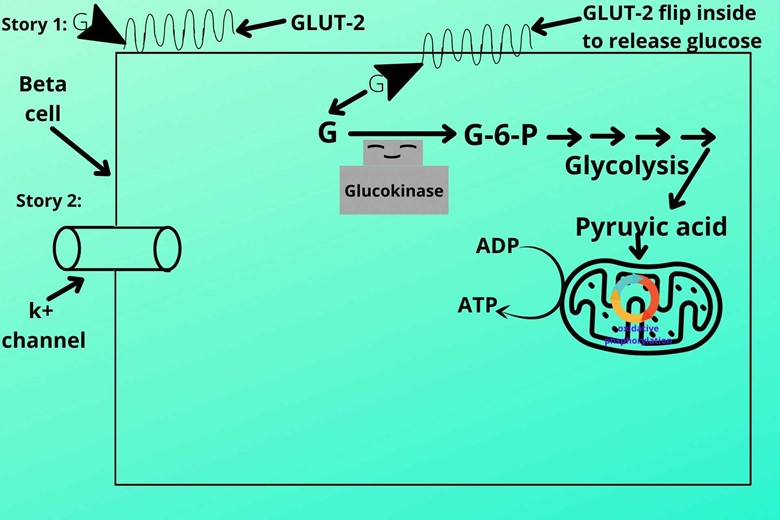
Potassium channels are present on the beta cell membrane which is open under normal conditions.
How it is open in normal conditions?
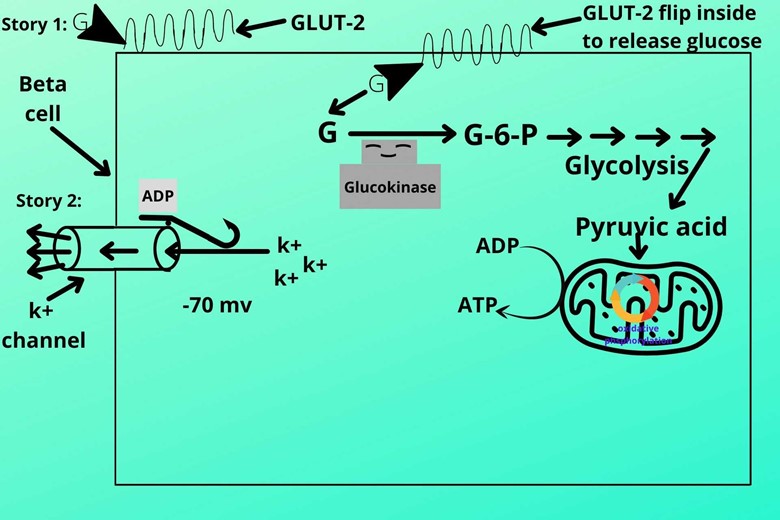
The structure of the k+ channel is such that it has a slit-like structure that has ADP on its tail part which makes him open under normal conditions.
When the channel is open it allows the potassium (k+) ions to escape, this makes the inner membrane more electronegative (polarized electronegative). Usually, the electronegativity remains -70 mv.
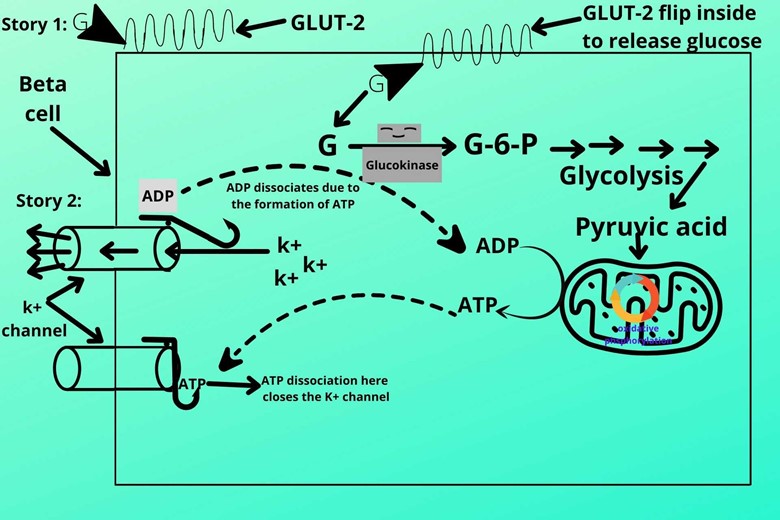
But when the ATP level rises the ATP sits at the head end of the slit-like structure and makes it heavy which automatically closes the k+ channel.
As ATP closes and opens the channel so we call it ATP sensitive potassium channel or ATP regulated potassium channel.
(VII). Retaining of k+ ions inside the cell:
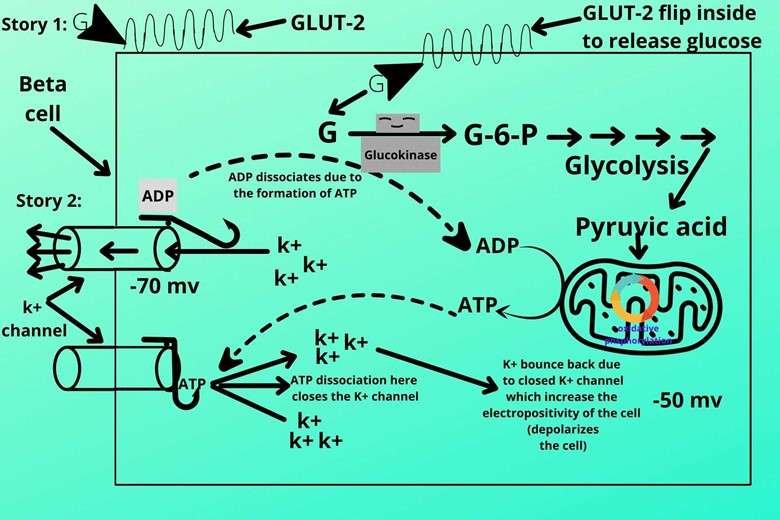
As the potassium channel closed the positive (k+) ions no more lose so the electronegativity of the cell decreases, in other words, we can say the cell becomes depolarized.
(VIII). Opening of ca+ (calcium) channel:
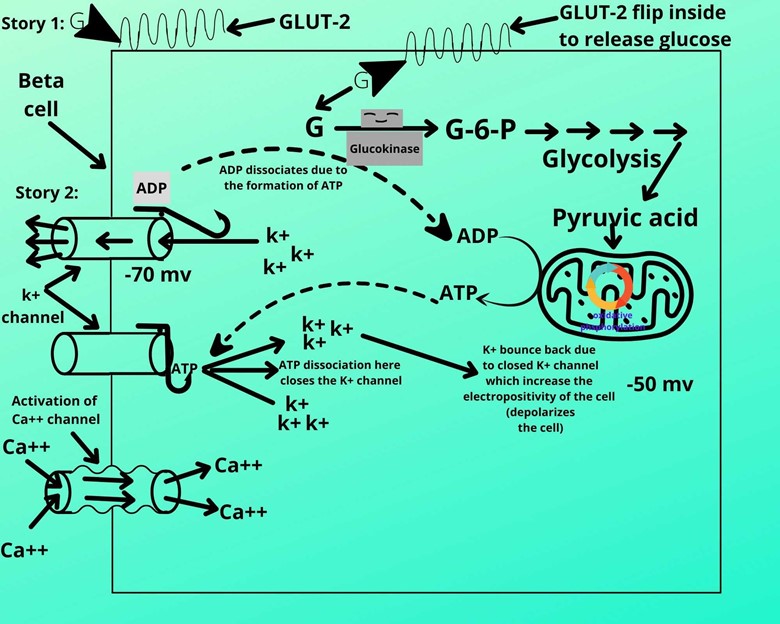
As the depolarization get decreases (the cell becomes less electronegative) the calcium channels are stimulated to open. These calcium channels are called voltage-sensitive channels because after retaining positive ions the electronegativity gets reduced which opens this channel.
(IX). Entrance of ca+ ion:
(X). Activation of secretory vesicles:
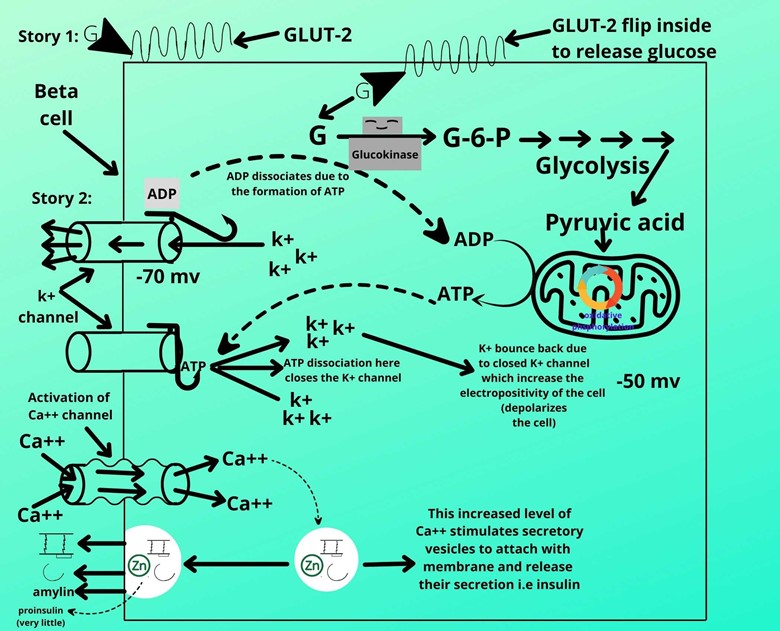
The entry of ca+ ions increases their concentration in the cell which stimulates the secretory vesicles to attach to the membrane of beta cells.
After attachment to the membrane, the vesicles will secrete their secretion which contains active or mature insulin, C-peptide, amylin, and very little amount of proinsulin.
This is the end of part 2 of the story and also it is the mechanism of insulin secretion or insulin release mechanism.
Is this all about today’s topic?
No, here it will be easy for you to understand one of the most important diseases diabetes mellitus type II.
I am sure you must know diabetes mellitus type I?
If you already know, I am still shortly defining this diabetes.
In type 1 diabetes the beta cells are destroyed due to they cannot produce insulin.
It’s all about type 1 diabetes.
Now coming back to diabetes mellitus type II.
Diabetes mellitus type II:
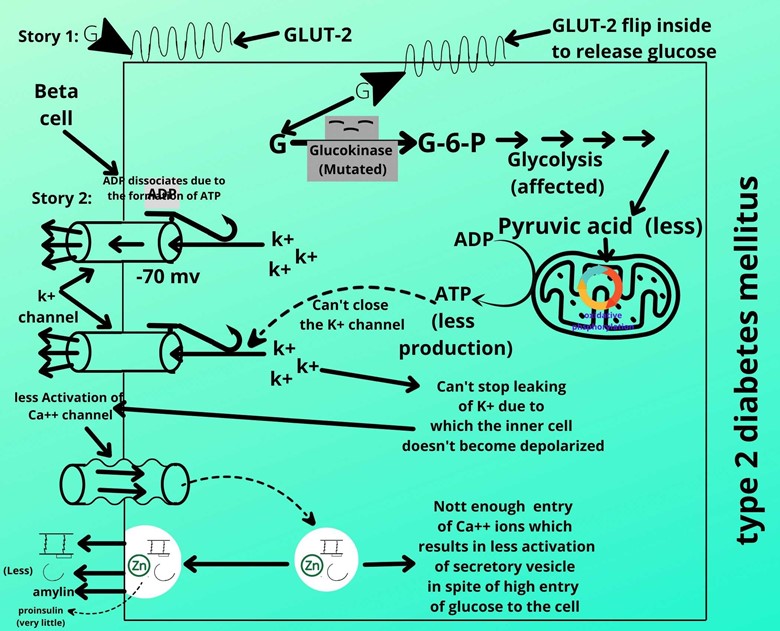
I will go back to the story part 1 for a better understanding. Did you remember the step?
Phosphorylation of glucose in which glucokinase enzyme converts glucose to glucose-6-phosphate.
In diabetes mellitus type 1 patients the glucokinase is mutated and it is not converting glucose to glucose-6-phosphate efficiently. It means due to not functioning well glucokinase is converting very few glucose molecules to glucose-6-kinase which further affects and reduces the conversion of ADP to ATP.
It means the potassium channel will also be affected?
Exactly, the potassium channel is not closing well due to the deficiency of ATP which couldn’t retain the ATP inside the cell leading to the leaking of potassium which couldn’t depolarize (reduce electronegativity) the cell inside.
I remember the next step which is the activation of the ca+ channel.
Correct Genius.
But here due to the leaking of potassium, the Ca+ channel does not open due to which the extracellular Ca+ cannot enter the cell. And you already know that the Ca+ entry activates the vesicle to secrete its secretion.
Though there is a lot of glucose entry but in response, enough insulin is not produced and this is basically type 2 diabetes mellitus in which the body doesn’t produce enough insulin for glucose. This type of diabetes mellitus type 2 patients suffer from MODY.
M MATURITY
O ONSET
D DIABETES MELLITUS
Y YOUNG PEOPLE.
So this type of diabetes is common in young people.
I think it is clear now.
All is clear now, Are there other groups of nutrients or factors that stimulate insulin secretion other than carbohydrates?
(A). Other groups:
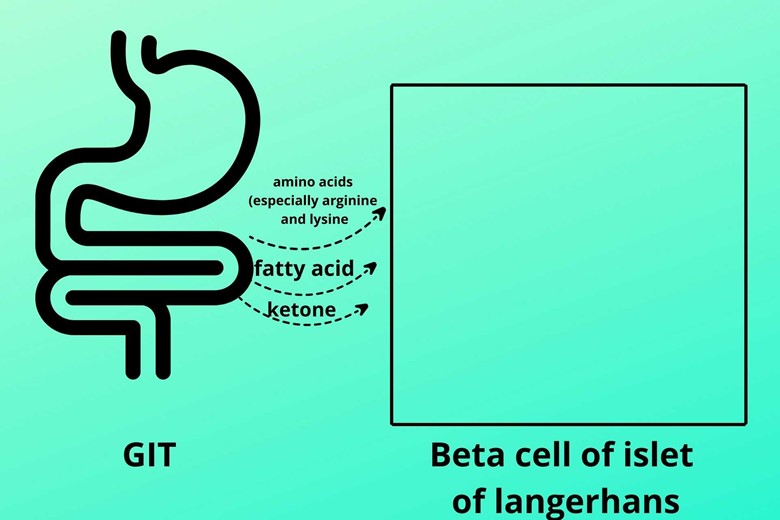
Yes, besides glucose the amino acid (especially arginine and lysine), fatty acid, and ketone stimulate the beta cells to secrete insulin.
But remember the master stimulator of insulin is glucose. The amino acids, fatty acids, and ketones activate insulin secretion as the glucose molecule did in the beta cells.
(B). INCRETINS:
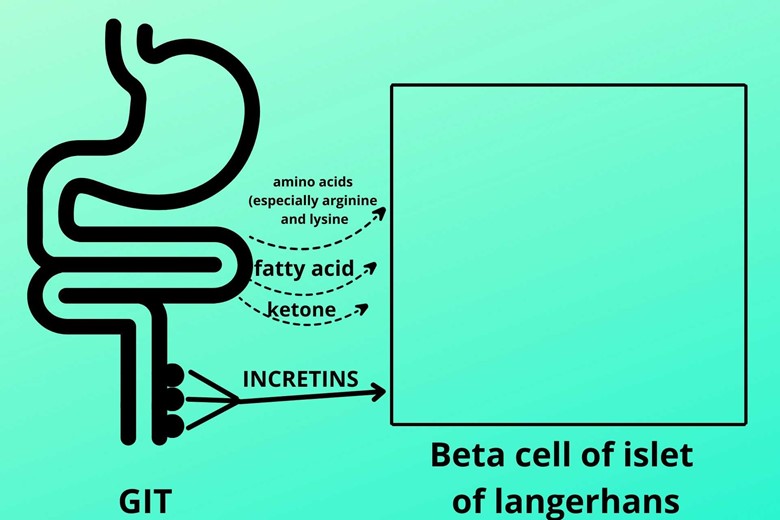
The walls of the GIT have epithelial cells that release a group of peptide hormones called INCRETINS which stimulates insulin secretion.
When it will be released?
Whenever someone loads their GIT with food the INCRETINS are released. INCRETINS are natural antidiabetic hormones that increase the insulin secretions
Other functions of INCRETINS.
- Secretion of insulin (already discussed)
- Inhibition of glucagon
- Inhibit, or slow down the activities and movement of the stomach (inhibit gastric emptying) to absorb the nutrients effectively.
- Produce satiety (feeling of satisfaction and not taking more food)
Now I want to tell you what hormones are INCRETINS.
That’s good. The first two INCRETINS hormones are most important because certain drugs contain GLP-1 to secrete more insulin.
- Glucagon-like peptide-1 or GLP-1 (it is different from glucagon, it is produced by the Gastrointestinal system while glucagon is produced by alpha cells of the islet of Langerhans)
- The gastric inhibitory peptide also called a glucose-dependent insulinotropic peptide (originally discovered to slow down the stomach movement but it has also other functions like insulin production, inhibiting and decreasing the release of glucagon, and satiety)
- Secretin
- Cholecystokinin
- Gastrin
(C). Glucagon:
Glucagon also stimulates beta cells to secrete insulin
How?
Glucagon binds with their receptor which is coupled inside with G-stimulatory protein which in turn stimulates adenyl cyclase where the ATP is converted to cAMP. The increased cAMP level leads to the release of insulin.
Glucagon-like peptide-1 or GLP-1 and Gastric inhibitory peptide receptors are also coupled with G-stimulatory protein which increases the rise in cAMP level and leads to insulin production.
Patients with Glucagonoma release much glucagon which will exhaust and damage the beta cells after some time which leads to type-II diabetes.
(D). Parasympathetic nervous system:
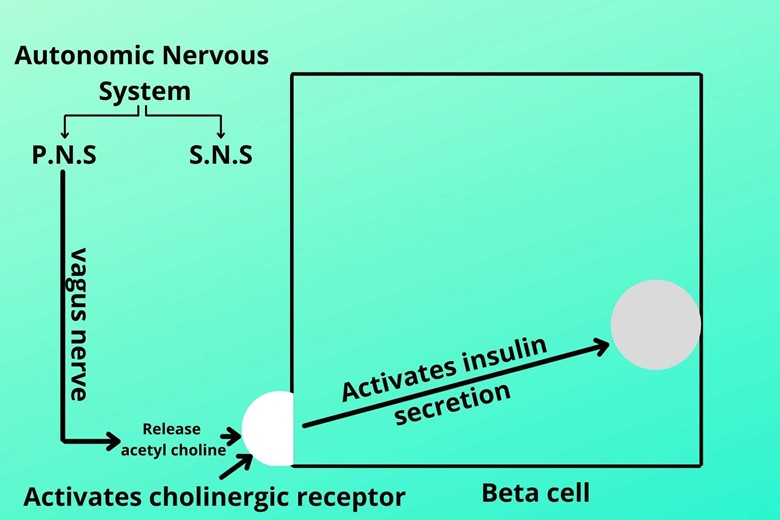
While taking food the vagus nerve of the parasympathetic nervous system is activated which releases the acetylcholine neurotransmitter which is then bound to their receptor called cholinergic receptor on beta-cell and activates insulin secretion.
In an emergency or stressful condition, the sympathetic nervous system inhibits insulin secretion so that the glucose doesn’t store in the liver and muscle cells and there is a continuous supply of glucose to the brain and central nervous system.
Now what is the time pattern of insulin secretion, I mean Is all insulin pre-prepared for the action?
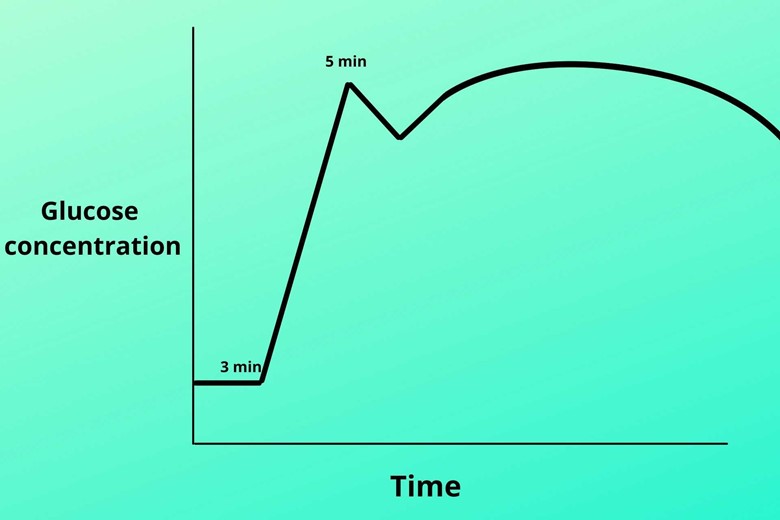
This is a good question indeed.
Insulin works in biphasic. It means when you take food after 3 minutes the insulin level starts rising rapidly and reaches to peak after 5 minutes, but after 5 minutes it gradually decreases. After some time again the insulin starts increasing until the glucose level becomes normal. So there are two peaks in the graph.
Why does this happen?
Because at the start there is pre-prepared (stored) insulin so the graph moves up rapidly, but when this bulk of pre-stored insulin is used the line gets down. Again after this, the new insulin synthesis starts and its synthesis continues until the glucose level gets to normal.
Can you tell the types of insulin?
There are three types of insulin that were named after their extraction from their source.
- Human insulin:
Insulin that is produced in humans is called human insulin as the name indicates.
- Pig or insulin:
Insulin that is derived from a pig is called pig or porcine insulin.
- Bovine insulin:
Bovine insulin is derived from a cow.
Which of these is closer to humans?
Porcine insulin is closer to humans because porcine insulin has a 1 amino acid difference from human insulin while bovine insulin has a 3 amino acid difference.
It was used for diabetes treatment but now both are taken out of the market because of their risk factor.
Can you tell us the half-life of insulin?
The half-life of insulin is 5-6 minutes. After secretion, it moves to the liver and acts there where it is then destroyed 60% and fewer amount moves to portal blood circulation. While the half-life of C-peptide is more which is about 30 minutes so it is a marker for insulin.
CONCLUSION/SUMMARY:
For better understanding, it is strongly recommended to read the whole article. But the important factor for releasing insulin is the ATP cycle stimulated by glucose molecules. In the whole process of Insulin synthesis, secretion, and function preproinsulin is converted into mature insulin.






1 thought on “Insulin synthesis, secretion, and function”